The Language of Life (Part 2): Beyond AlphaFold—From 'Reading' a Fold to 'Writing' a Function
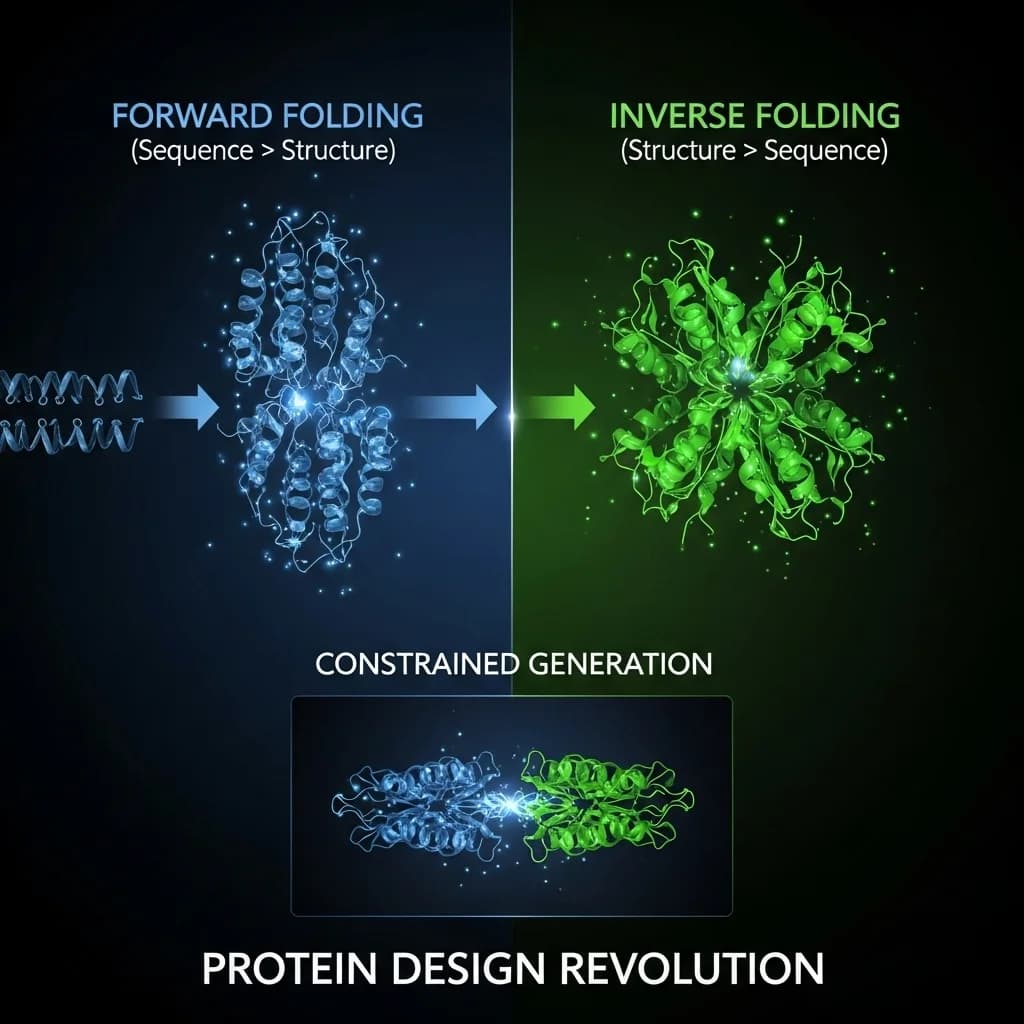
Table of Contents
- AlphaFold's Revolution: The Era of "Reading"
- The Limitation of "Reading" for Drug Design
- The "How": Generative Models for 3D Structure
- "Writing" Function: "Constrained Hallucination" and "Inpainting"
- Table 2: SOTA Generative Architectures in the Humanome.ai Platform
- Conclusion
AlphaFold's Revolution: The Era of "Reading"
In Part 1, we established how Protein Language Models (PLMs) learn the 1D "grammar" of protein sequences. Now, we address the true core of function: the 3D structure.
It is impossible to overstate the impact of AlphaFold. It brilliantly solved a 50-year-old grand challenge in biology: the "forward folding" problem. Given a 1D amino acid sequence, AlphaFold can predict its 3D structure with astounding accuracy.
This was a revolution in "reading" the language of life. For the first time, we could reliably see the "meaning" (structure) of any given "sentence" (sequence). But for drug discovery and protein design, this is only half the battle.
The Limitation of "Reading" for Drug Design
As drug designers and R&D leaders, we rarely start with a random sequence. We start with a problem: a disease target we need to bind, an enzyme we need to create, or a function we need to perform.
Our question is not, "What does this existing protein do?" Our question is, "Build me a new protein that does this specific thing."
This requires solving the Inverse Folding Problem: Given a desired 3D structure (which embodies a function), generate the 1D amino acid sequence(s) that will fold into it.
The "How": Generative Models for 3D Structure
At Humanome.ai, we take this a step further. We don't just inverse-fold an existing structure; we invent the target structure itself, de novo. Our generative models "dream" or "hallucinate" novel protein backbones, built from the first principles of biophysics they have learned.
Our technical stack for this includes two main classes of generative models:
Diffusion Probabilistic Models (DPMs)
Models like RFdiffusion and Chroma have become SOTA for de novo backbone generation.
How it works (The "Denoising" Process): These models are trained by taking all known protein structures from the PDB, adding "noise" until they are just a random "gas" or "cloud" of C-alpha atom coordinates in 3D space. The model then learns to reverse this "diffusion" process. To generate a new protein, we start with pure noise and ask the model to "denoise" it, step-by-step, applying the learned physical rules of protein folding. The result is a stable, physically-realizable protein backbone that has never been seen in nature.
Flow-Matching Models
Newer, more efficient architectures like OriginFlow and ADFLIP represent the cutting edge.
How it works: These models learn a continuous, deterministic path from noise to structure, making generation faster. They achieve SOTA performance in generating diverse, "designable" structures and are particularly adept at handling complex, all-atom contexts, including multi-chain complexes and bound ligands.
"Writing" Function: "Constrained Hallucination" and "Inpainting"
This is the technical core of how we design function. We do not generate random, (though beautiful), new folds. We generate folds for a specific purpose. The method is known as "Constrained Hallucination" or "Inpainting".
This is our in-silico "sculpting" process:
-
Define Function: We digitally define the "business end" of the protein. This is the active site—a small constellation of residues in a precise 3D geometry. This could be a catalytic triad for an enzyme, a receptor-binding motif, or a pocket to coordinate a metal ion.
-
Constrain Generation: We "freeze" this functional motif in 3D space.
-
"Hallucinate" Scaffold: We task our generative model (e.g., Chroma) to "inpaint" or "hallucinate" around this fixed motif. The model "dreams up" a novel, stable protein backbone whose sole purpose is to hold those functional residues in that exact, pre-defined, active conformation.
-
Sequence Design: Once we have this de novo 3D backbone "scaffold," we use a SOTA inverse folding model (like ProteinMPNN) to determine the optimal amino acid sequence that will fold into it.
This "constrained hallucination" approach allows us to decouple function from evolutionary baggage. Natural proteins evolved for survival, not to be ideal therapeutics. They are "messy"—often large, multi-domain, and riddled with allosteric sites and evolutionary spandrels.
Our de novo scaffolds are the opposite. They are minimalist, hyper-stable, and "clean." They are built from first principles to do one job perfectly. This makes them the ideal canvases for next-generation therapeutics, as they are designed for high stability and minimal off-target interactions.
Table 2: SOTA Generative Architectures in the Humanome.ai Platform
| Architecture Type | SOTA Example(s) | Primary Task (The "How") | Humanome.ai Application |
|---|---|---|---|
| Masked LM (Transformer) | ESM-2, ProtT5 | Bidirectional context analysis (MLM) | "Learning the Grammar" / Extracting rich biophysical embeddings |
| Autoregressive LM (Transformer) | ProGen2, ProtGPT2 | Unidirectional next-token prediction | Unconstrained de novo sequence generation |
| 3D Diffusion (Polymer) | RFdiffusion, Chroma | Denoising 3D coordinate "noise" into stable backbones | De novo "hallucination" of novel protein scaffolds |
| 3D Flow-Matching | OriginFlow, ADFLIP | Efficient, continuous generation of 3D structures | High-speed design of functional binders and multi-chain complexes |
| GNN Inverse Folding | ProteinMPNN | Predicting sequence from a given backbone | "Threading" the amino acid sequence onto our de novo designed backbones |
| E(3)-Equivariant Diffusion | EDM, 3D-EDiffMG | Denoising atom types/coordinates in 3D space | De novo generation of small molecules inside a 3D pocket (see Part 3) |
Conclusion
We have moved from "what does this protein do?" to "build me a protein that does this." This is the core of generative drug design.
Now that we can design the 3D protein "lock," the next question is clear: How do we design the perfect small molecule "key" to fit it, atom by atom? That is the subject of Part 3.
#AlphaFold #proteinDesign #inverseFolding #diffusionModels #drugDiscovery